Buffer solutions & reagents
Product Description
A buffer solution is a solution that can neutralize the addition of a small amount of added acid, alkali or dilution, characterized by its ability to keep its pH value mostly unchanged. The reagents used to prepare the solution are also called buffer agents. Buffer solutions are generally composed of weak acids and weak salts or weak bases and weak base salts with the same ion effect. For example: acetic acid-sodium acetate, ammonia-ammonium chloride, sodium bicarbonate-sodium sulfate, borax-boric acid, and potassium dihydrogen phosphate-disodium hydrogen phosphate can all be formulated into buffer solutions, which can be divided into several types according to different uses.
Our Buffer Solution Applications
Primary pH standard buffer solution for pH reference reagent determination
Primary pH standard buffer solution for pH reference reagent determination
Primary pH standard buffer solution for pH reference reagent determination
This buffer is used for the determination of pH reference reagents and the calibration of high-precision pH meters. Equivalent to IUPAC Class C. The reagents of this level use the double hydrogen electrode of the liquid-free junction cell to measure the pH value. The accuracy of the method is ± 0.005 pH value. This reagent is generally produced by the state-controlled manufacturers and tested by the Chinese Academy of Metrology.
Standard pH buffer solution for measuring reference reagent pH value
Primary pH standard buffer solution for pH reference reagent determination
Primary pH standard buffer solution for pH reference reagent determination
A solution for the preparation of pH reference reagents for the calibration of pH meters. Equivalent to IUPAC’s D level, each reagent manufacturer uses the primary pH reference reagent as a standard, and uses a double hydrogen electrode with a liquid junction cell to measure its pH value. The accuracy of the method is ± 0.01 pH value.
Standard buffer solution for pH determination of chemicals
Standard buffer solution for pH determination of chemicals
Standard buffer solution for pH determination of chemicals
pH standard buffer solution is a set of standard solutions, its pH range is from 1.0-13.0, there is a standard solution every 0.1 pH in the range of pH 1.0-10.0; there is a standard solution every 0.2 in the range of pH 10.0-13.0. This solution can be used as a standard for the determination of pH value by colorimetric and a standard solution for the determination of the color range of acid-base indicators. The accuracy is ± 0.03 pH value. It is not as accurate as the pH reference reagent, so it is not suitable for the calibration of pH meters. The buffer volume is low, and it is not suitable for constant complex o metric titration buffer solution.
Buffer solution for biochemical research
Standard buffer solution for pH determination of chemicals
Standard buffer solution for pH determination of chemicals
This kind of buffer solution is a special buffer solution for biochemical analysis. It is generally used to control the pH value in the research process of DNA, protein and other biological macromolecules. Considering the changes of DNA and protein, the pH range is between 5.0-8.0 pH.
Our Buffer Solutions
Sodium 3-(N-morpholine)propanesulfonate
1,4-Piperazine diethanesulfonic acid disodium salt
1,4-Piperazine diethanesulfonic acid disodium salt

CAS: 71119-22-7
3-morpholine-2-hydroxypropanesulfonic acid
1,4-Piperazine diethanesulfonic acid disodium salt
Sodium 3-morpholine-2-hydroxypropanesulfonate

CAS: 68399-77-9
Sodium 3-morpholine-2-hydroxypropanesulfonate
3-bis(2-hydroxyethyl)amino-2-hydroxypropanesulfonic acid
Sodium 3-morpholine-2-hydroxypropanesulfonate

CAS: 79803-73-9
3-bis(2-hydroxyethyl)amino-2-hydroxypropanesulfonic acid
3-bis(2-hydroxyethyl)amino-2-hydroxypropanesulfonic acid
3-bis(2-hydroxyethyl)amino-2-hydroxypropanesulfonic acid
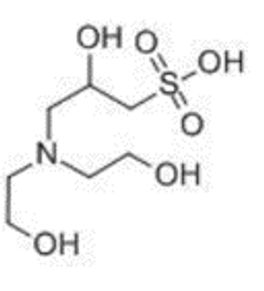
CAS: 68399-80-4
4-(2-Hydroxyethyl)-1-piperazinepropanesulfonic acid
3-bis(2-hydroxyethyl)amino-2-hydroxypropanesulfonic acid
3-bis(2-hydroxyethyl)amino-2-hydroxypropanesulfonic acid

CAS: 16052-06-5
4-(2-Hydroxyethyl)piperazine-1-2-hydroxypropanesulfonic acid
4-(2-Hydroxyethyl)piperazine-1-2-hydroxypropanesulfonic acid
4-(2-Hydroxyethyl)piperazine-1-2-hydroxypropanesulfonic acid

CAS: 68399-78-0
4-Hydroxyethylpiperazineethanesulfonic acid
4-(2-Hydroxyethyl)piperazine-1-2-hydroxypropanesulfonic acid
4-(2-Hydroxyethyl)piperazine-1-2-hydroxypropanesulfonic acid

CAS: 7365-45-9
ADA monosodium salt
4-(2-Hydroxyethyl)piperazine-1-2-hydroxypropanesulfonic acid
CAPSO monosodium salt

CAS: 7415-22-7
CAPSO monosodium salt
N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid monosodium salt
CAPSO monosodium salt

CAS: 102601-34-3
N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid monosodium salt
N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid monosodium salt
N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid monosodium salt

CAS: 70331-82-7
N-tris(hydroxymethyl)methanoic acid-2-hydroxypropanesulfonic acid
N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid monosodium salt
N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid monosodium salt

CAS: 68399-81-5
N-Ethyl-N-(3-propanesulfo)aniline sodium salt (ALPS)
N-tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid
N-tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid

CAS: 82611-85-6
TRIS-HCl
N-tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid
Tetracalcium phosphate

CAS: 1185-53-1
Tetracalcium phosphate
Piperazine-N,N-bis(2-hydroxypropanesulfonic acid)
Tetracalcium phosphate
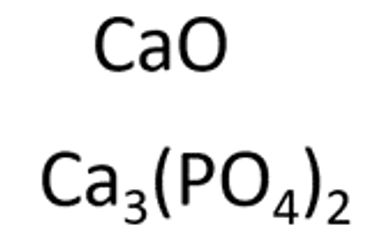
CAS: 1306-01-0
Morpholineethanesulfonic acid
Piperazine-N,N-bis(2-hydroxypropanesulfonic acid)
Piperazine-N,N-bis(2-hydroxypropanesulfonic acid)

CAS: 4432-31-9
Piperazine-N,N-bis(2-hydroxypropanesulfonic acid)
Piperazine-N,N-bis(2-hydroxypropanesulfonic acid)
Piperazine-N,N-bis(2-hydroxypropanesulfonic acid)

CAS: 68189-43-5
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.